TriApex Laboratories has two clinical subsidiaries, Chengdu Aesthetic Medical and TriApex Med, which design and operate ophthalmic clinical trials, provide registration and consulting services. These teams work with the preclinical team at TriApex Laboratories to accelerate preclinical to clinical translation.
These clinical subsidiaries have more than 10 years of clinical experience. Their commitment is to achieve the highest quality, success-oriented clinical trial design in order to produce the most reliable clinical research results.
 Service Advantages
Service AdvantagesProfessional experience of clinical teams
Chengdu Beauty Medical has been dedicated to the clinical trials of pharmacology drugs and medical devices for over ten years. The core members have more than ten years of experience in phase Ⅰ-Ⅲ ophthalmology clinical trials. Our team members receive ophthalmology training (OCT, FFA and BCVA) and have frequent hospital visits to learn clinical procedure and treatment tools.
 Clinical Service
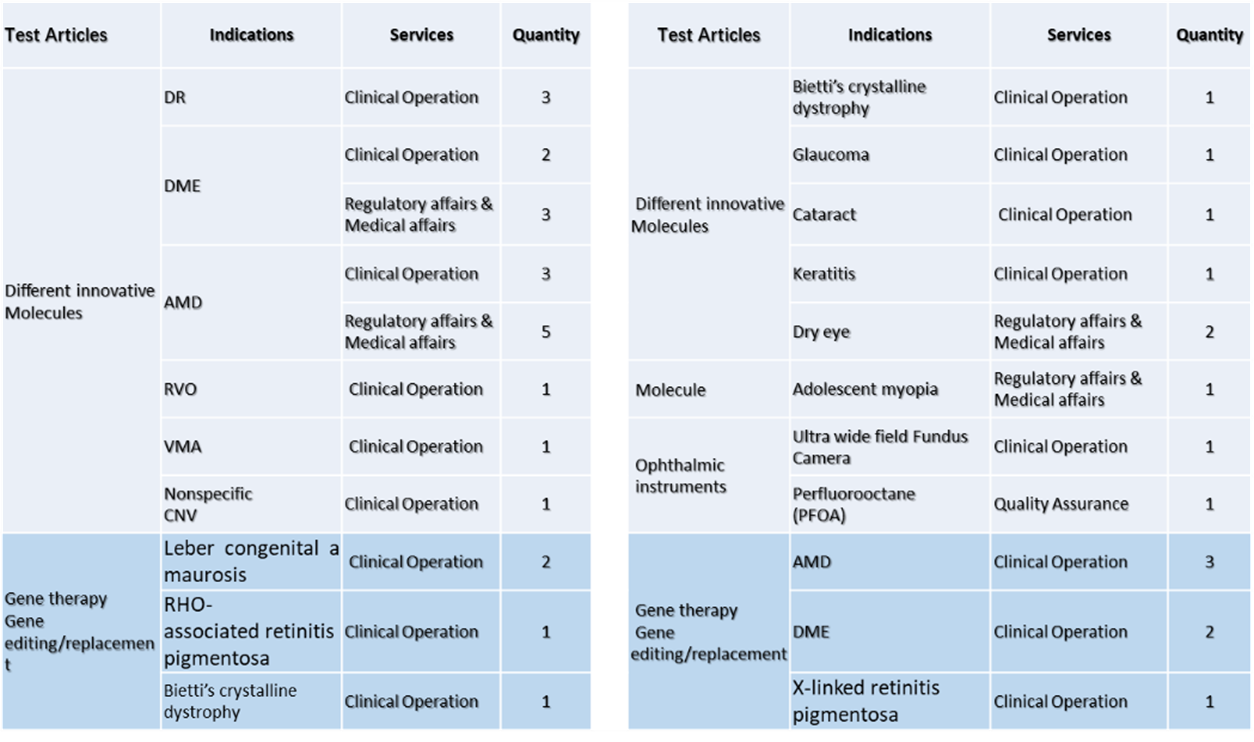
Clinical Service-Various services: regulatory affairs, phase I, II, III clinical trials.
-Various test articles: small molecule, antibody, gene therapy, etc.
-Various indications: corneal diseases, cataract, fundus diseases, hereditary retinal diseases, etc.
-Various products: eye drops, ocular injections, medical devices, etc.
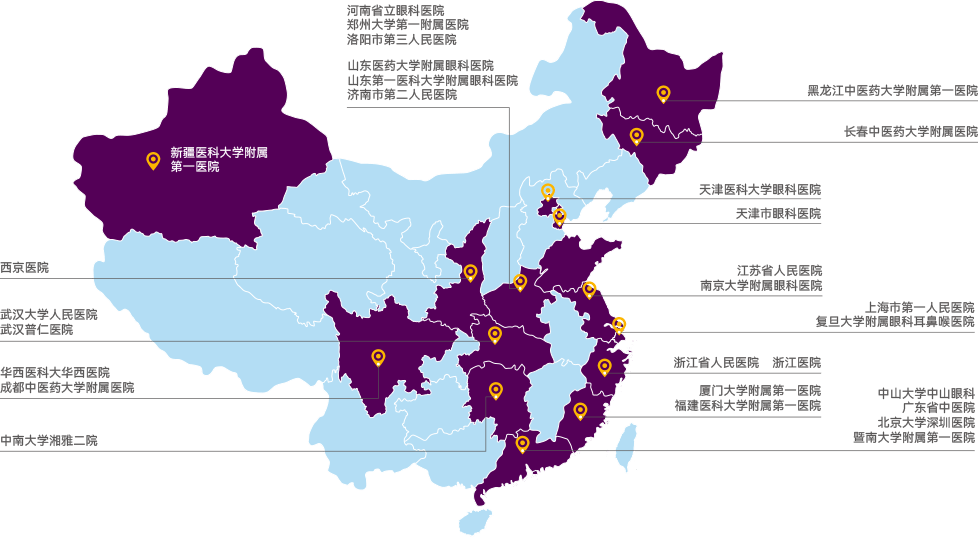
TriApex has established extensive and stable collaborations and partnerships with high-level hospitals and enterprises.