
TriApex DMPK team is committed to providing pharmacokinetic research services for PK R&D studies at various research stages, including leading compound discovery and optimization, PCC identification, and other phases, to facilitate drug R&D from non-clinical to clinical stages.
 Service Advantages
Service Advantages1.Comprehensive Research
Proficient in conducting various pharmacokinetic studies to support the development of new chemical entities (including siRNA) and biologics (including cellular & gene therapies).
Various animal species including non-human primate, dog, mini pig, mice (including tumor-bearing mice), rat, rabbit, guinea pig, golden hamsters.
2.Expertise in Various Drugs
New chemical entities (including oligonucleotides), antibodies, ADCs, cellular & gene therapies, etc.
3.Excellent Project Operation System
Certified standardized experimental system: high quality and high reproducibility of experimental data.
Professional project management system: high project operation efficiency and short operation cycle.
 Service Capacities
Service Capacities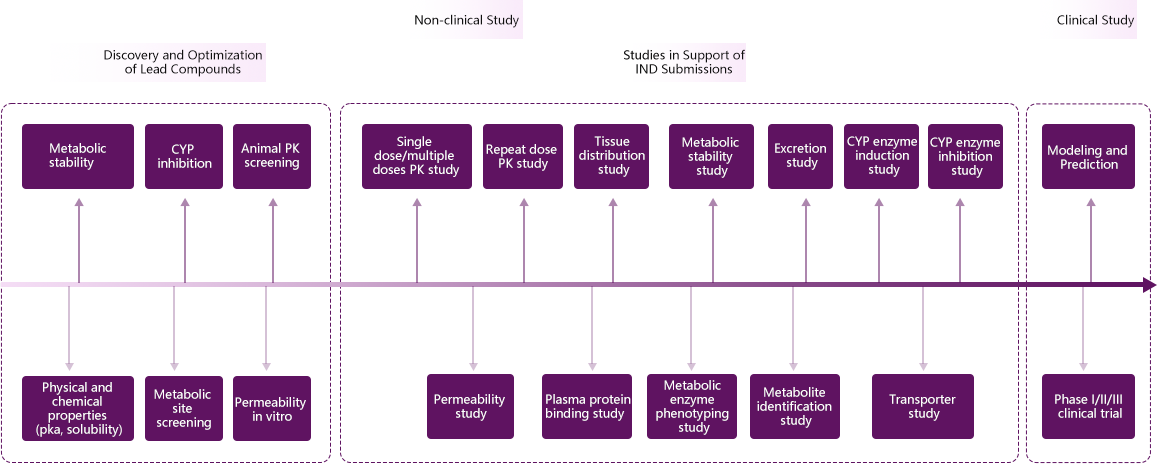
1.Study on Physical and Chemical Properties (1)Solubility study (2)Lipophilicity study (3)Solution stability study 2.Absorption (1)New Chemical Entities Permeability study: Caco-2 cell model, MDR2-MDCK cell model Absorption studies at one dose and multiple doses: rodent, non-rodent Single dose and repeat dose absorption studies: rodent, non-rodent (2)Biologics Single dose and repeat dose absorption studies: rodent, non-rodent 3.Distribution (1)Chemical Entities Blood plasma partition ratio: whole blood and plasma of various species Plasma protein binding: equilibrium dialysis, ultrafiltration, electrophoretic mobility shift assay (EMSA) Tissue distribution study: rodent (2)Biologics/Cellular &Gene Therapies BioDistribution 4.Metabolism (1)Metabolic stability study: liver microsome, liver S9, mixed liver cells of various species (2)Metabolite identification study: metabolite identification in vitro and in vivo (3)Metabolic phenotyping identification study: human liver microsome, recombinant human CYP450 isoenzyme (chemical inhibition method, recombinant enzyme method) (4)UGT phenotyping identification 5.Excretion (1)Excretion study: bile, urine, feces 6.Drug-Drug Interaction (1)Transporter study (substrates and inhibitors) Efflux transporters: ABC transporter (P-gp, BCRP) Uptake transporters: SLC transporter (OATP1B1, OATP1B3, OAT1, OATP3, OCT1, OCT3, MATE1, MATE2K) (2)Metabolic enzyme inhibition study CYP450 Isoform: 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 3A4 and 2E1 (3)Metabolic enzyme induction study CYP450 Isoform: CYP3A4, CYP2B6, CYP1A2 |
 Project Experience
Project ExperienceTriApex has successfully conducted more than 200 pharmacokinetic studies of new chemical entities (NCEs) and accelerated the development of these products.
Compound 1 is a small interfering RNA (siRNA) intended for the treatment of hyperlipidemia. We designed and conducted pharmacokinetic studies in vitro and in vivo to support IND application of the product. The studies are as follows:
Single dose pharmacokinetics study in rats and monkeys by subcutaneous injection
Study on tissue distribution in rats and plasma protein binding in vitro
Stability studies in serum and liver S9
Metabolite identification study in vitro and in vivo
CYP450 metabolic enzyme phenotyping study
Metabolic enzyme induction and inhibition study
Excretion study
Transporter study
The above studies fully demonstrated the pharmacokinetic characteristics of Compound 1, which facilitated IND application. The IND application has been approved.
 Guidelines
Guidelines1. Technical Guidelines for clinical Single and multiple dose escalation pharmacokinetic studies of chemotherapeutic innovative Drugs (No. 58 of 2021), NMPA, December 2021
2. Technical Guidelines for Clinical Pharmacology Research of Innovative Drugs (Draft), NMPA, August 2021
3. Technical Guidelines for Drug Interaction Studies (Pilot) (No. 4 of 2021), NMPA, Jan 2021
4. Technical Guidelines for Clinical pharmacokinetic Studies of Chemical Drugs, March 2005
5. FDA M9 Biopharmaceutics Classification System-Based Biowaivers, 2021.05
6. FDA In Vitro Drug Interaction Studies - Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry, 2020.01
7. ICH M10 Bioanalytical method validation and study sample analysis, 2023.01
