
As an emerging technology in the biomedical field, organoids and Organ-on-a-Chip show great application potential in the fields of basic scientific research, translational medicine and drug R&D, especially in developmental biology, regenerative mechanism, precision medicine and pharmacodynamics studies of drug safety and efficacy evaluation, etc. Organoids also make it possible to harvest cells that more closely resemble natural human development for cell therapy.
 Introduction of Services
Introduction of Services1.Based on the construction of iPSCs-Cardiac Organoids drug screening/testing.
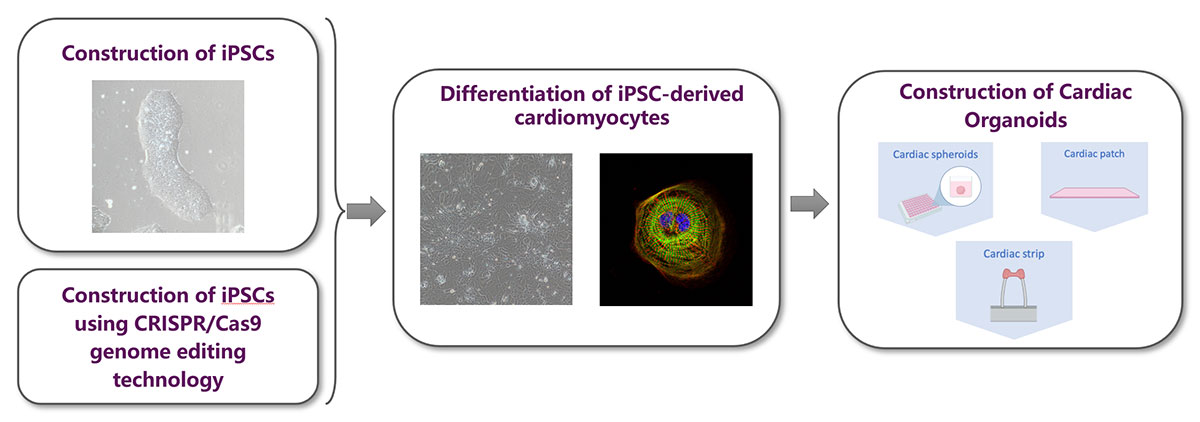
iPSCs-cardiac organoids-based in vitro high-throughput screening/detecting can be combined with traditional animal effects/toxic syndrome detection to provide research data that helps improve transformative value.
With the rise of New Modality, cardiac organoids will play an important role in in vitro efficacy and safety evaluation.
2.Based on the construction of iPSCs-Retinal Organoids and drug screening/testing.
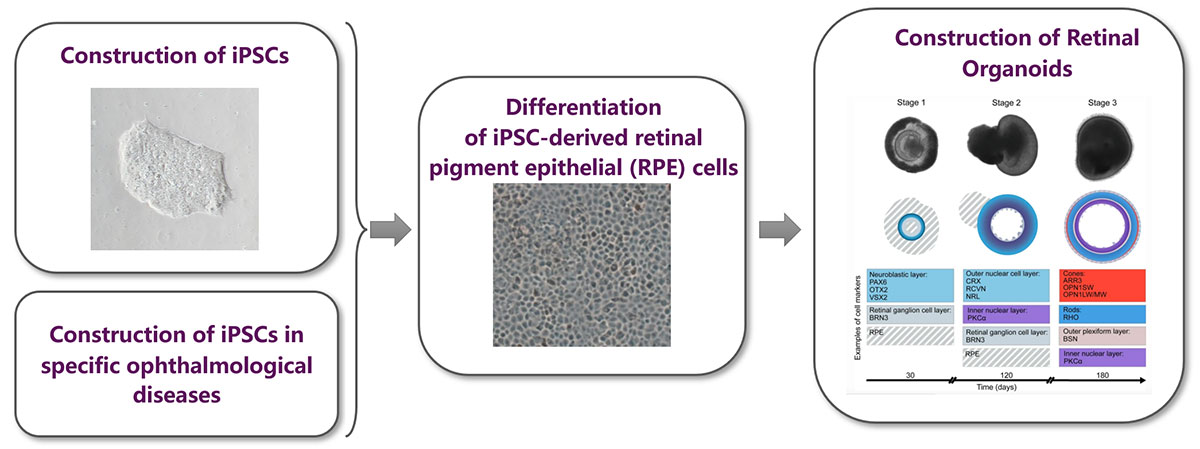
Culture retinal organoids based on iPSCs-RPE and iPSCs-RGC functional cells, which can be sued to simulate some specific ocular diseases in vitro.
Combining with traditional ocular disease models, it provide research data that can help improve the transformative value.
With the rise of New Modality (e.g. AAV gene delivery carrier), Retinal Organoids will play an important role in in vitro efficacy and safety evaluation.
1.iPSCs product development strategy service
TriApex can provide following services based on the characteristics of iPSCs products to lower the possible risks in the product development process:
Clarify development demands, market prospect analysis and competition assessment;
Clarify development demands, market prospect analysis and competition assessment;
Early risk assessment of iPSCs models;
Research on activity and safety under laboratory/Non-GLP conditions.
2. Method development and confirmation service
TriApex is the leading domestic CRO in non-clinical research and clinical research, and work with clients to obtain the scientific and regulatory-compliant results during the method validation and preclinical research of iPSCs products. We can provide services including, but not limited to:
Confirmation of clinical demands and method validation;
iPSCs product R&D and quality specifications (SOPs) guidance.
3. Preclinical/laboratory research service
TriApex can provide testing service of iPSC-derived cardiomyocyte, liver cell and retinal pigment epithelial cell therapies or compounds.
4. Medical writing service.
TriApex can provide report writing services for in vitro research on iPSC-derived cardiomyocytes, liver cells and retinal pigmentation cells, and write partial or all study reports for the submission of certification materials and academic journals.
