
The“Translational Medicine and Biomarker Research”team is centered on“target-biomarker”research and utilizes advanced technological platforms to offer data-driven scientific support throughout the drug development process. From early-stage Mechanism of Action (MOA) study, pre-clinical to clinical translational research, to Proof of Mechanism (POM) and Proof of Concept (POC) of clinical trials. We also focus on the purpose of biomarker studies to meet the clinical demand, and engage in co-development of companion diagnostics (CDx) to accelerate the drug development process.
 Service Advantages
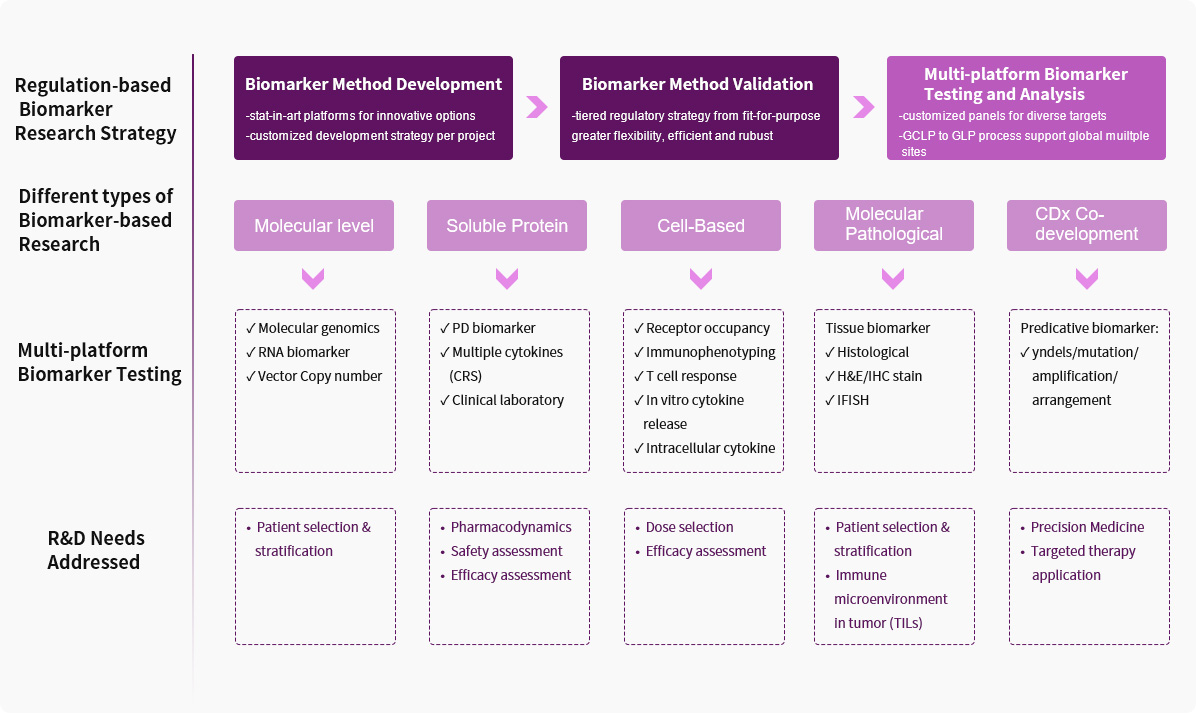
Service Advantages1. Diverse Validated Biomarker Methods
We have completed methodological development and validation for biomarkers at different levels and using different detection platforms, including multi-gene sequencing, multiplex cytokine analysis, immunophenotyping, receptor occupancy, and protein phosphorylation. Additionally, we can provide a wider range of methodological choices in accordance with project requirements, enabling faster detection and more effective cost control.
2. Extensive Project Experience
We have participated in the exploration and translation of biomarkers in over 40 targets and different indications in preclinical and clinical research. The types of drugs involved include small molecule targeted drugs, antibody drugs (monoclonal antibodies, bispecific antibodies, etc.), gene therapy, cell therapy, oncolytic viruses, mRNA vaccines, etc. In particular, we possess a wealth of exploratory research and cooperative experience in the field of Immuno-Oncology field.
3. Multidisciplinary Research Team
Our Translational Medicine and Biomarker research team comprises experts from a variety of backgrounds, including molecular biology, molecular pharmacology, oncology and immunology, clinical pharmacology, and bioinformatics, providing data-driven translational medicine support.
 Service Capacities
Service Capacities1.Soluble biomarkers
Validated multiplex panels:
-Human Inflammatory Cytokines: IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13,TNF-α
-Human Cytokines: GM-CSF, IL-1α, IL-5, IL-7, IL-12/IL-23p40, IL-15, IL-16, IL-17A, TNF-β, VEGF-A
-Human Chemokines: Eotaxin, MIP-1β, Eotaxin-3, TARC, IP-10, MIP-1α, IL-8, MCP-1, MDC, MCP-4
-Human Th17: IL-17A, IL-21, IL-22, IL-23, IL-27, IL-31, MIP-3α
-Human/Mouse TGF-β: TGF-β1, TGF-β2, TGF-β3
-NHP Cytokines: IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-10
Validated biomarkers:
-Human VEGF (VEGF-A), TARC, total IgE, free IgE, IL-18, Granzyme B, Perforin, Periostin, Calprotectin, MASP-2, sBCMA, DKK1, IGF-1, CSF-1 etc.
2.Cellular biomarker detection
Immunophenotypic analysis:
-Human T/B/NK/Treg: CD3, CD4, CD8, D16, CD56, CD25, CD127, CD19
-Human T cell subset: CD45, CD3, CD4, CD8, CCR7, CD45RA, CD45RO
-Human T/B/NK/Treg/NKT panel: CD45,CD3, CD4, CD8, CD19, CD16, CD56, CD25, CD127, CD1d,TCR Va24
-Human T cell activation panel: CD45, CD3, CD4, CD8, CD69, CD25, CD28, 41-BB, Ki67
-Human B cell activation panel: CD45, CD19, CD69, CD86
-CAR-T Immunophenotyping
-Intracellular cytokine: CD45, CD3, CD4, CD8, TNF-α, IFN-γ, IL-2, IL-4
Receptor occupancy:
-PD1, PDL1, Lag3, IL4R, CD47, CD38, CD40, SIPRα, PDL1 x CD47, EGFR x CD3, CD112R, CD39
