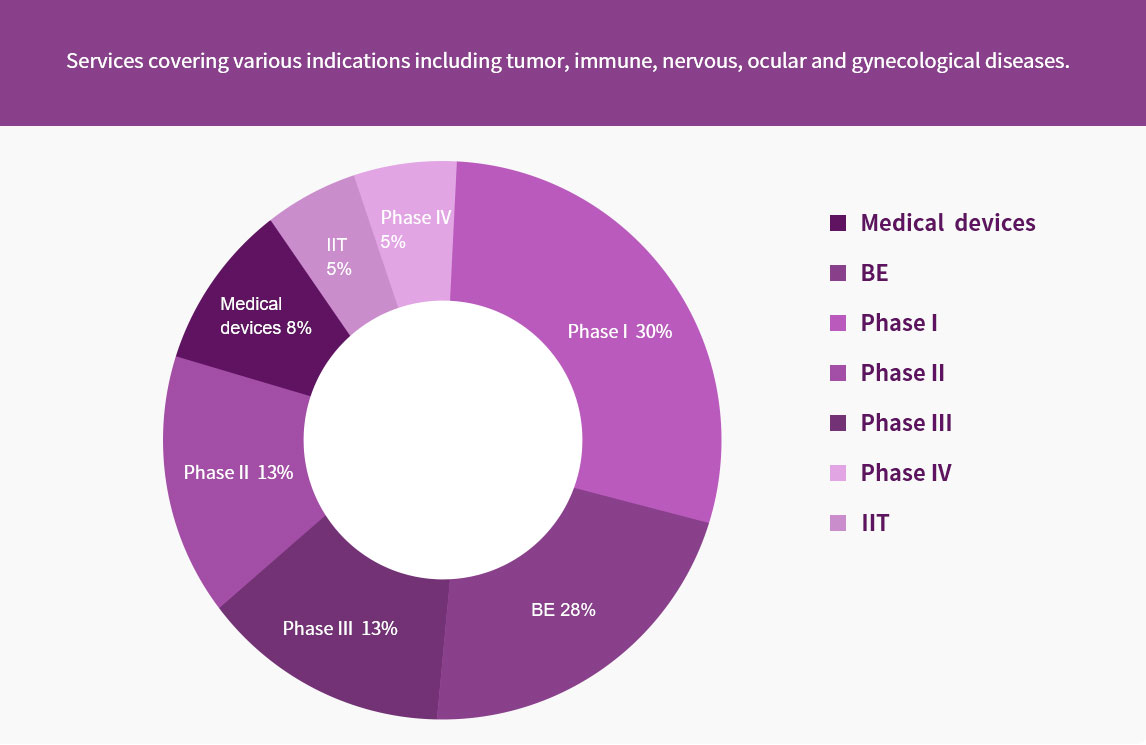
The clinical data management (DM) team has professional background highly in line with the requirements of clinical trials. The DM team, of which most members are medical-related majors such as medicine, pharmacy and biostatistics, provides professional, efficient, accurate and regulatory-compliant services for all stages of data management. The DM team also provides a full range of ICH guideline-, GCP- and regulatory-compliant data management services from protocol review to database lock. At present, we have completed over 100 studies, and has rich experience in BE/I-IV /RWE data management.
 Service Capacities
Service CapacitiesFull services from program initiation to submission:
-Clinical trial protocol review
-Data management plan (DMP) development
-CRF (Case Report form) /eCRF design
-CRF Completion Guideline (CCG) development
-Data Validation plan (DVP) development
-Database design and maintenance
-Database System Configuration and testing
-Edit check programming and testing
-User account and access administration
-User training
-Data entry/external data import
-Data management progress report development
-Data verification and query management
-Medical coding
-SAE reconciliation
-Data quality control
-Data review
-Database lock
-Data management report development
-External data management
-Project document management
 Project Experience
Project Experience