The medical affairs team of TriApex, a professional team with clinical medicine background and clinical study experience, is committed to providing you with professional and high-quality medical affairs services, including study design, protocol development, and medical monitoring. TriApex is ready to provide the case-by-case development strategy and scientific and feasible study protocols according to product characteristics through combining with domestic and international research overview, technical status and development trend, and accurately recommend the optimal selection of indications and clinical research routes.
 Service Capacities
Service CapacitiesDevelopment strategy and protocol design
TriApex is ready to provide the case-by-case development strategy and scientific and feasible study protocols according to product characteristics through combining with domestic and international research overview, technical status and development trend, and accurately recommend the optimal selection of indications and clinical research routes.
Medical monitoring
Medical training
Subject enrollment review
Protocol deviation review
Medical support
CRF medical review
Medical review of clinical data, including medical support in AE, laboratory data, medical history, drug combination
Medical writing
Clinical trial protocol (phases I to IV, BE, IIT)
CRF
ICF
Investigator’s Brochure
Clinical study report
Clinical study literature reference and overview
Medical papers, study report writing, etc.
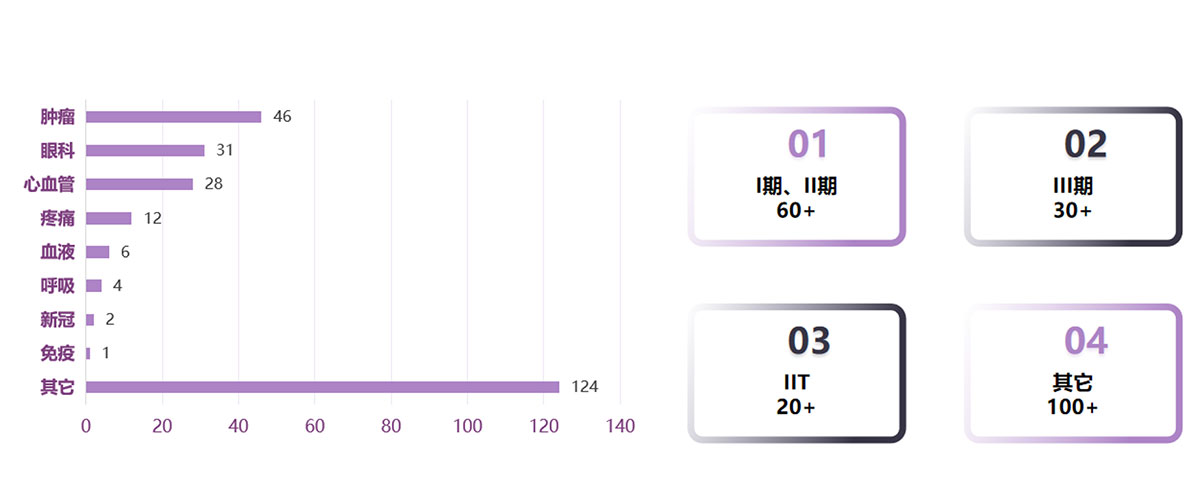
 Project Experience
Project Experience