TriApex, with a comprehensive SOP system for project operation, makes sure that the processes of clinical research projects are conducted in compliance with ICH-GCP and NMPA GCP regulations, and efficiently obtains high-quality clinical research data. We are committed to providing services that fully meet the demands of international and domestic sponsors in clinical trials of pharmaceutical products.
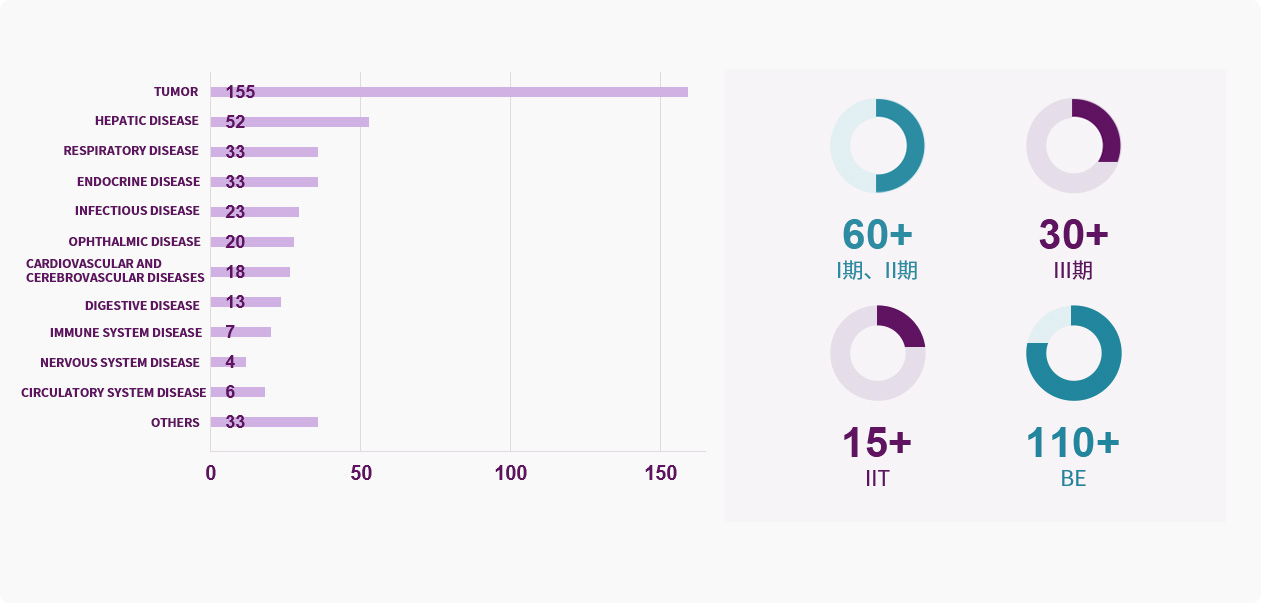
 Service Capacities
Service Capacities-Clinical research center screening (selection of investigators and research centers);
-Preparation of basic materials and documents for clinical trials;
-Initiation of clinical trials (organization of investigator meetings, ethical declaration, genetic resource application, contract signing with research center, etc.);
-Clinical trial monitoring (SDR, SDV, gap analysis);
-Clinical research center COV (Close Out Visits);
-Cooperate with Clinical research center in collaborative inspection and quality control.
 Project Experience
Project Experience